One-pot Synthesis of N-substituted Secondary Amines
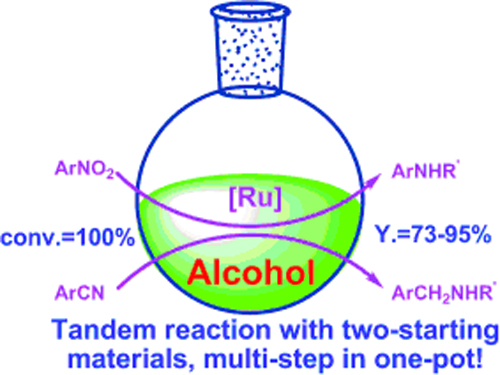
One-pot synthesis of N-substituted amines from nitro and nitrile compounds and alcohols.(Image by SHI Feng et al.)
The R&D Center for Green Chemistry and catalysis of the Lanzhou Institute of Chemical Physics (LICP), CAS, has developed one-pot synthesis of N-substituted secondary amines from nitrobenzenes/benzonitriles and alcohols. N-Alkylated amines with various structures could be synthesized with 73–95% isolated yields. In this process, multiple steps were realized in one-pot with high efficiency.
The work presents a versatile and simple method for the synthesis of N-substituted amines in excellent yield and high efficiency from nitro and nitrile compounds with alcohols. It offers a clean and economic way for the synthesis of N-substituted amines.
Amines and their derivatives play critical roles as building blocks, functional linkages, and key moieties in peptides, polymers, and many natural products and pharmaceuticals. The previously reported methods of the synthesis of N-substituted amines normally involve multiple steps. This work has realized the amination of alcohols to secondary amine from nitro or nitrile compounds directly.
The work has received support from the National Natural Science Foundation of China and “Hundred Talents Program” of the CAS. The detailed report has been published in Chem. Eur. J.(Chem. Eur. J. 2011, 17, 2587 – 2591).